Care for steel protection
The world of steel
Rust is the biggest enemy of metals. It forms where metal reacts with oxygen and moisture. A salty environment, high temperatures or the presence of chemicals, accelerate and intensify the rusting process.
Rust is a form of corrosion. Corrosion includes the loss of metals, but also any adverse effect on materials as a result of environmental conditions.
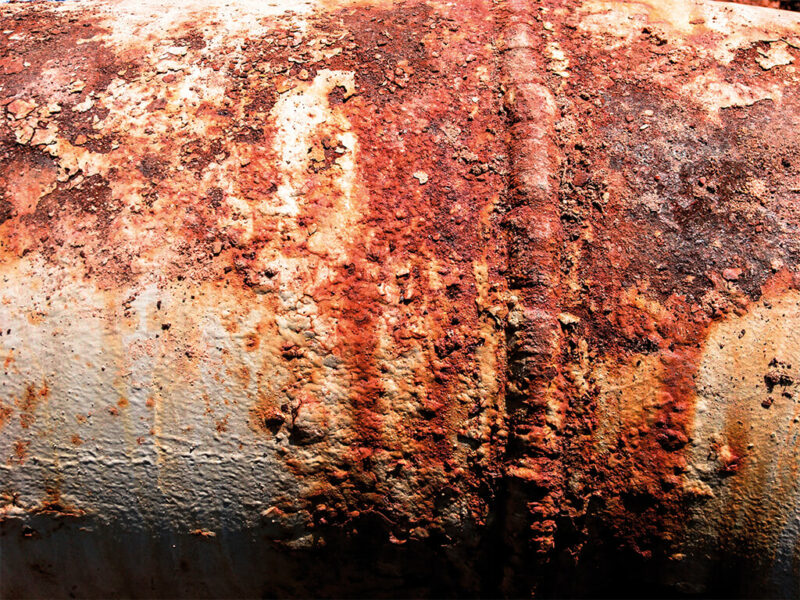
Rust is a reddish-brown, flaky coat that appears on metal. Nothing more and nothing less.
Rust is the common name for a very pernicious compound technically called iron oxide. Rusting is a specific kind of corrosion, which applies to metals that contain iron. Rust starts to form when a drop of water comes into contact with an iron or steel object. In combination with oxygen the metal starts to corrode.
The technical term for this process is called oxidation. The iron or steel will inevitably rust to an extent that depends on the length of time and amount of exposure to oxygen and water. Sometimes it can take days, months or even years depending on the intensity of exposure.
Rust is a flaky powder that replaces the strong iron, for example in pipelines. The iron not only loses its strength, but also its smoothness and electrical conductivity, and may develop holes.
Surface preparation
The removal of contaminations, imperfections, rust and mill scale, plus creating a roughness profile is called surface preparation. Surface preparation is defined as ‘the cleaning or treating of the metal surface prior to the application of a surface coating’. Good surface preparation ensures the best possible bond between the surface to be coated and the coating to be applied.
How do you get a fully rusted surface clean again? That’s where MontiPower® comes in. Our advanced technology with handheld, semi-automatic or automatic machines is designed to clean and create roughness. The unique method of the Bristle Blaster® is an amazing innovation in surface preparation.

83% inadequate surface preparation
Corrosion is an electrochemical reaction, whereby oxygen and water cause iron to rust or copper to turn green. Corrosion causes enormous economic damage – no less than approximately 4 percent of the Gross National Product. Parts that are affected by corrosion must be treated or replaced. For transport pipes this can quickly run up to a few hundred thousand euros. Corrosion also holds enormous risks. A pipe under pressure can give way in an eroded weak place or sheet piling can break up.

Adhesion is a function of
peak height and peak density
The solution
The solution for corrosion consists of removing one or more of the 3 required components – water, oxygen or the electrochemical reaction. Traditional coatings for metals, such as bitumen, polyethylene (PE) systems, polypropylene (PP) systems, and epoxy powder coating (FBE) or epoxies, cannot prevent water and / or oxygen from reaching the metal. Therefore for instance, transport pipes use cathodic protection to stop the electrochemical reaction, whereby an electric current stops the ionization of the iron. This requires energy and constant monitoring of the protection. That is why rust and corrosion prevention require an effective surface preparation method.
 Portuguese (Portugal)
Portuguese (Portugal) Dutch
Dutch Spanish
Spanish French
French German
German English
English