Dr. Prepper No. 2

Dr. Prepper’s special guest: Tom Swan from MTEST
Common myths about salts, chlorides and coatings
Dr. Prepper regularly invites surface preparation, coating and corrosion protection specialists to share their knowledge and experience. In this edition, meet Tom Swan, General Manager of MTEST (MontiPower–Test Equipment & Specialty Tool), located in Houston, Texas.
Tom has led MTEST since its inception in 2009 and has many years of experience working with coatings, corrosion control and integritiy management. He is a recognized industry leader and expert on testing and inspection equipment.
Tom is a member of the Houston Coating Society, SSPC and NACE and highly appreciated in the Houston area and beyond for his knowledge and sensible advice.
Today, Tom will share with us his thoughts on certain popular beliefs – aka common myths – concerning salts, chlorides and coatings.

The regular RED PAPER from MontiPower®. Full of nice to know – and need to know – facts about the most optimal surface preparation since 1987.

A lot has been written about salts and chlorides over the years. Much of it is true, sometimes it’s misleading, but sometime it’s just downright false.
Today Tom and Dr. Prepper will cut through the marketing hype to sort out fact vs. fiction and share the truth.
WARNING
TO PROFESSIONALS WORKING IN SURFACE PREPARATION, CORROSION PREVENTION AND COATING INDUSTRY:
Look beyond the marketing to separate fact from fiction.


DR. PREPPER: “Tom, you mentioned many articles and specifications interchange the term “salt” and “chloride”. Why is that an issue?”
TOM: While most of the salts on metal or concrete surfaces are generally chloride salts, not all salts contain chloride. In the field, the only way to determine “salt” is to measure conductivity and approximate the total salts or total dissolved solids (TDS). The only way to measure the chloride anion is to use a chloride-specific test.
To determine which test is best for a project, we need to understand the specific concern of “invisible surface contaminants”.
What constitutes a Salt?
DR. PREPPER: “What is the difference between a Chloride and a salt, Tom?”
TOM: Well, the first step is to understand what constitutes a salt. Salts are often referred to as molecules. The fact is, salts are not actually molecules, but should really be called “formula units”. This makes sense if we look at the lattice structure of a salt crystal.
Let’s examine a sodium chloride (NaCl) crystal (Figure 1).
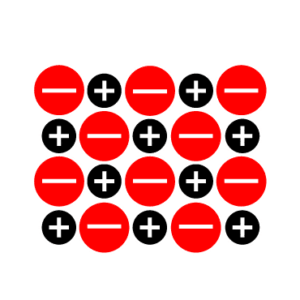
Figure 1 shows that each chloride ion is bound to four (six for face-centered cubic lattice) sodium ions by strong electrostatic charges called ionic bonds. Each sodium ion is also bound to four other chloride ions by ionic bonds.
Since no single ion is attached to any other ion, this is not a molecule but a lattice of 1 chloride anion (Cl–) to 1 sodium cation (Na+) or NaCl. Salts are typically composed of a metal atom (cation) and a non-metal atom (anion) joined by ionic bonds. Other salts contain two non-metal atoms (ammonium chloride [Na4Cl]) or two metal atoms (calcium plumbate [CaO3Pb]).

DR. PREPPER: “Our industry has been flooded with articles on chloride and salts that suggest some are worse than others when it comes to osmotic blistering (e.g., chloride, sulfate, and nitrate are bad).
Can you break down a few terms used in those articles, Tom?”
TOM: Sure thing.
Solution
- A solution is composed of a solvent and a solute. For present purposes, the solvent is water and the solute is the substance on the surface under the paint that is water soluble.
Osmosis
- This is a colligative property of solutions that depends on the number of particles or ions in a given volume of solvent, not on the mass or type of the particles or ions.
- Osmosis is independent of the chemical nature of the solute, but very dependent on the solvent. What is dissolved can be ions, solvents, or organics such as sugar.
Osmotic Pressure
- Osmotic pressure depends on the number of dissolved particles or ions, not the chemical nature of the solute.
- So, the type of solute is not important. On the other hand, the type of solvent is important. This will be described in greater detail below.

So let’s examine 5 common myths or popular beliefs in our industry that we hear repeated often.
Myth #1: ‘Chloride salts pull water through membranes’
Many industry articles suggest some salts containing chloride anions are hygroscopic, and are, therefore, worse than others in terms of inciting osmotic blisters. According to the myth, the hygroscopic chloride anion, generally in the form of NaCl, pulls water through the coating causing an osmotic cell to grow.
TOM: THIS IS FALSE.
The truth is that chloride salts don’t pull water through the coating. The solution on one side of the coating cannot directly detect what is on the other side unless there is a breach or hole in the coating. Moreover, the salt normally cited as a problem, NaCl, is not particularly hygroscopic when compared to many other salts.
The miraculous behaviour of water molecules
A coating is considered semipermeable, which means it will let small molecules (water) pass through, but will not allow larger molecules (anions and cations) to pass through. When water is on the surface of the coating, small amounts migrate through the coating as individual molecules. When these molecules reach the substrate underneath the coating, if the surface is clean, the molecules of water continue on their way. When the water reaches the surface, if it encounters soluble salts, the water will dissolve any soluble salts or other water-soluble materials to form a solution.
The dissolved particles lower the vapor pressure of the solution which prevents the liquid from leaving the solution and creates a driving force (osmotic pressure) from the side with higher vapor pressure (the liquid outside the coating) toward the side with the lower vapor pressure (the solution beneath the coating). Osmotic pressure is determined by the relationship of the two solutions separated by the semipermeable membrane (the coating).

DR. PREPPER:
So Tom, you’re saying that the number of ions in the solution, or molar concentration, determines what the osmotic pressure is, not the composition of ions?
TOM: That’s right, Dr. Prepper. Soluble salt ions such as Na+ and Cl- or soluble organics such as sugars or solvents. 1-3 The important thing to note is: the solute which is dissolved has no bearing on osmosis. It does not even have to be a salt. Again, what matters is the number of particles that are dissolved. The osmotic theory that treats this blistering of the coatings is true only at extreme dilution, so the theory is valid for this discussion.
While the nature of the solute has no bearing on osmosis, it does have a bearing on the creation of a corrosion cell. If the solute is ionic, the liquid under the coating will be an electrolyte and will form a corrosion cell. The solute that is dissolved will affect the strength of the corrosion cell. If salts present in the solution render the pH acidic, the corrosion cell will be more corrosive than if the solution is basic.
Myth #2: ‘Chemical Additives are Necessary to Remove Water-Soluble Salts’
DR. PREPPER: This leads into my next question. We often hear in our industry that the only way to completely remove water-soluble salts is with chemical additives. This popular belief leads us into a discussion of the role of chemicals in reducing water-soluble salts or chloride contamination.
TOM: THIS IS FALSE
In most situations the use of high-pressure water is sufficient to remove salts to a level low enough to meet the extraction criterion. It is important to use good-quality water to prevent further contamination of the surface. Most potable water is acceptable, generally firewater or sea water is not. The objective is to have the surface meet the extraction criterion at the end of the wash cycle.
Pressurized water cleans a surface better than just pouring a bucket of water over it.
The energy imparted to pressurized water helps break the surface tension of the water so that it more effectively wets the surface. Typically, higher purity water will dissolve more salts. Adding a surfactant (rinse aid) the wash water can increase its efficiency the same way that adding soap to a dishwasher helps clean the dishes.
As the wash water quality decreases, or the surface is corroded or pitted, surface cleaners (rinse aids) can help remove the salts. However, if the wash water is too contaminated, not even the presence of the chemicals will help clean the surface. In some cases, if the wrong cleaner is used, they may even increase the contamination. After washing, the surface should be tested to measure the amounts of salts on it.
All cleaners work generally in the same way
DR. PREPPER: CAN YOU EXPLAIN HOW CLEANERS WORK ON SURFACES?
TOM: To remove the salts, the solvent (water) needs to reach the solute (the salts). It is necessary for the cleaner to reduce the surface tension of the water to better access the salts. The surfactant in the rinse aids, along with the pressurized water, allows the water to better reach the salts which are then pulled into the solution by the water. This also helps to remove insoluble particles and along with light oil and grease.
This is why most salt removal products require the use of high-pressure water. Generally, the higher the pressure, the more efficient the wash.

The only way to be certain the surface is salt free, even after cleaning, is to run a conductivity test.
Myth #3: ‘Only Acid-Based Cleaners will Remove Chloride, Sulfate, or Nitrate’
DR. PREPPER: I read a few articles suggesting the only effective way to remove chloride-, sulfate-, or nitrate-containing salts is to use acid-based cleaners.
TOM: THIS IS A MYTH.
There are several products on the market that remove salts from steel. Acidic, neutral, and basic cleaners can all be used effectively.
What we learned from the Kennedy Space Center Study
A two-year study conducted by the Kennedy Space Center compared four cleaners formulated to remove chloride ions. The cleaners were all off-the-shelf products that are generically called chemical rinse aids (CRAs) and were already in use for washing down aircraft.
These CRAs included acidic (initial were pH = 3.5), neutral, and basic (initial pH = 9.0) cleaners.
The researchers rinsed various metal coupons and let them dry in a beach exposure. Comparisons were made between coupons rinsed with demineralized water, seawater and some which were not rinsed. The researchers found that the pH of the cleaner had no effect on how well they removed salts from steel to stop atmospheric corrosion.
Alkaline cleaners are generally based on amines. They are non-ionic and contribute little or no conductivity to wash water. Some, such as HoldTight®102 are 100% volatile and will evaporate from the surface with the water. Should any residue remain from the rinse aid, a corrosion cell will form, but as the solution becomes more basic, it inhibits or reduces corrosion.

Myth #4: ‘Testing for Chloride, Sulfate, and Nitrate Ions Ensures a Salt-Free Surface’
DR. PREPPER:
This common belief essentially states that if you want to ensure a salt-free surface it is sufficient to test for the presence of the three most common salts. What is your opinion?
TOM: Since chloride, sulfate, and nitrate ions represent the majority of salts present, they are generally a good indicator of salts left on a particular surface, and indeed many specifications require testing for one or more of these ions.
If the main concern is chloride-containing road salts, testing for chloride will probably find the majority of these ions – it also may not.
Acid-based cleaners, for example, contain other salts, which may contaminate the surface during cleaning. However, they do not contain Cl-, SO4-, or NO2-ions. With non-ionic cleaners, this is not a concern.
As we mentioned earlier, the only way to be certain your surface is salt-free, even after cleaning, is to run a conductivity test.
If there is no conductivity, there are no chloride, sulfate, nitrate or any other ions present on the surface. And that is precisely why the European standards and the International Marine Organization (IMO) standards require conductivity testing.
Myth #5:
DR. PREPPER:
I’ve heard that ‘Amine Based Cleaners Do Not Remove Salts, They Mask them’. Is that true?
It is often stated that some cleaners may mask salts so they are not detected during testing.
This could be either
FACT OR FICTION,
depending on the chemistry of the cleaner
TOM: IT DEPENDS. Whether a cleaner masks certain salts really depends on the chemistry of the cleaner.
Some inhibitors work by forming a barrier layer on the surface so oxygen and water cannot easily migrate through the barrier. A common example is the use of phosphoric acid (H3PO4) or phosphates to form iron phosphate on the surface.
Phosphating steel is an accepted practice. In general terms, this involves use of an acid to react with or etch the steel to create a water-insoluble salt coating.
Other basic inhibitors could form barriers of iron oxide or iron hydroxide (FeHO2) by reacting with a water-soluble iron salt to form a water-insoluble salt layer. The barrier would mask salts if they were present on the steel underneath the barrier.
Amines, which are derivatives of ammonia (NH3), are weak bases and often cited as a film-forming product that can mask salts. Stating it this way is misleading, however; some amines are formulated to create a stable hydrophobic film on the metal surface to repel water. These types of amines are often used in the water treatment industry to inhibit corrosion. Again, the amine barrier could mask the salts under the barrier if the salts were present on the steel.
Amines can be formulated to have many different properties, however, including being aromatic. Because they are non-ionic, products such as HoldTight®102 are aromatic amines and will not leave a film that masks salts on surfaces. They will not add any salts to the cleaned surface or form any impenetrable hydroxyl layer on the steel.

Tom’s conclusions:
DR. PREPPER:
Thanks for breaking down fact and fiction for us, Tom. Any final words?
TOM: Common myths or popular industry beliefs exist because of different experiences and different agendas, whether for science or for marketing reasons. Just because it is in print does not make it true. Many coating and corrosion articles are not based on original research, but rather on previous articles.
Often, parts of articles or technical papers are quoted out of context, which misrepresents the meaning of the original text.
Errors from one article get passed on to subsequent articles!
Some articles are a mix of personal opinion, practical observation, marketing hype with little or no professional peer review. If we say it often enough, myth twisted into truth.
DR. PREPPER: It sounds like those articles can be deceiving. But knowledge is power.
TOM: That’s right! It’s time to dust off that old chemistry book. When references are given, take the time to check them out. Don’t believe something just because someone said it in a lecture or printed it in an article.
Speaking of which;
Here are our references:
- C.Hare, JPCL 10 (2007): p.77
- C.Hare, JPCL 2 (1998): pp. 45-63
- C.Hare, JPCL 3 (1998): pp. 17-34
Download Dr. Prepper No. 02
You can also download the complete
Want to learn more about MTEST and coating?
MTEST
Tom Swan
5750 N Sam Houston Prky E
Suite 1016
Houston, TX 77032
Office: (281) 359-2215
Cell: (281) 300-9435
tswan@m-testco.com
 Portugiesisch, Portugal
Portugiesisch, Portugal Niederländisch
Niederländisch Spanisch
Spanisch Französisch
Französisch Deutsch
Deutsch Englisch
Englisch